|
Graphite Parts for CZ Crystal Pulling |
Fixtures for Semiconductor Processes |
|
Graphite Parts for Water Processing LPE Boats: Graphite Boats and other parts for LPE (liquid phase epitaxy) to produce LEDs (light emitting diodes) such as GaP and GaAs. Plasma CVD Parts: Plasma CVD Susceptors, Electrode Chambers, Ion Implantation Chambers, Sputtering Electrodes, MOCVD Susceptors and other applications. As a low atom number material, graphite has a strong resistance to ions or plasma. Under Tokai Carbon's stringent guidelines, graphite is highly purified. Consequently, it is ideally suited for plasma CVD parts. |
|

- Good electrical conductivity and high thermal conductivity
- Low thermal expansion resulting in high thermal shock resistance
- Strength at high temperatures
- Chemical inertness and non-wetting to glass and most metals
- Self lubricity
- Appropriate machinability

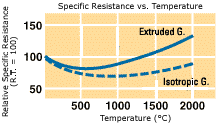
Relative specific resistance
One of the most useful characteristics of graphite is its ability to carry electric
current. Its resistivity alightly decreases when temperature rises up to 400 to 600 but
increases, though slightly, over this temperature range. The resistivity of graphite can
be controlled by modifying raw materials and manufacturing process to meet broad
requirements.
|
|

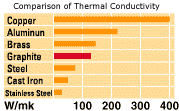
The expansion of graphite is considerably lower than that of practically all other materials. The high thermal shock resistance of graphite results principally from the low coefficient of thermal expansion and good thermal conductivity. The thermal shock resistance is shown in the following formula.
R=K.S/E
K: thermal conductivity (W/mK)
S: tensile strength(MPa)
a: coefficient of thermal expansion
( x10-6 /°C)
E: Young's modulus(GPa)

|
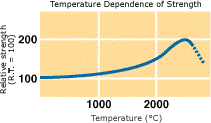
The mechanical strength of increases as temperature rises and the strength will be approximately double from room temperature to about 2,500°C. Compressive Strength The flexural strength and compressive strength have roughly the above correlations. |

|

|
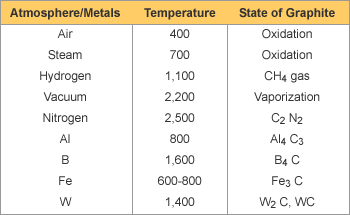
Graphite is resistant to most acids and alkali except for strong oxidizing media like concentrated nitric acid, etc and media to create lamellar compound like concentrated sulfuric acid, strong alkali and bromine etc. Graphite is chemically stable under normal conditions. But at high temperatures, it will react with some atmosphere and metals as shown in the left. Graphite does not ordinarily adhere to molten glass and most molten metals. |
|